Plan, execute, and manage your training lifecycle effortlessly with Learn-IQ. Tailored to meet your organization’s needs and compliance with regulatory standards like GBP, cGMP, FDA, MHRA, and ALCOA Plus.

A good Learning and Training Management software will help you

Right from identifying training needs to tracking, Learn-IQ takes care of all the stages of employee training lifecycle. It is agile in competency assessment and management.
Identify → Plan → Execute → Evaluate → Track
Learn-IQ ensures that all training activities are performed as per regulatory requirements to ensure competence development with complete audit trails.
Learn-IQ allows you to evaluate trainees using online & offline assessments. Generate question papers to print or choose to send online to trainees. All evaluations can be registered on the system for tracking.
With a flexible system as Learn-IQ, trainee assessment is flexible and traceable.
Follow security enforcement policy in line with the Electronic Records & Electronic Signatures rules set by regulatory authorities for compliance & data security.

Automatically schedule training sessions by specifying the session’s course, date, time, etc., & reduce time & effort of training coordinator.

Provide process-centric training in the proper version, restrict revision, and generate list of employees trained in both the versions.
Generate job responsibility report, training schedule reports, and many more to analyze the training strategy and make amendments.
Reduce time spent in making repetitive entries through quick group assignments according to the subjects, topics, and levels of training.
Training coordinators and management personnel can check individual and group training details instantly with the available drilldown dashboards.
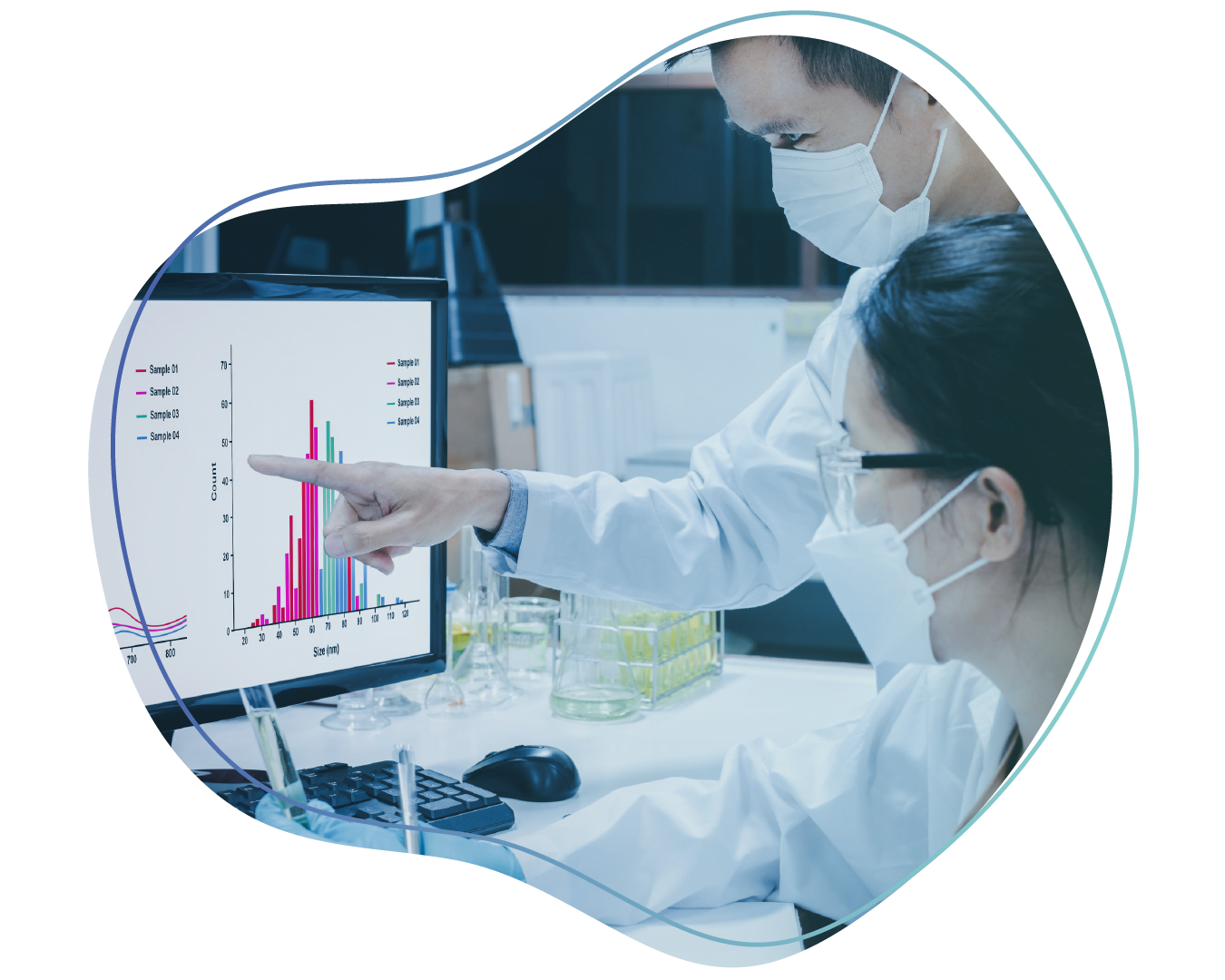
In life sciences and other regulated industries, it is imperative to have a GxP compliant training system. It should meet various regulations related to FDA, MHRA, ALOCA Plus, and other regulatory bodies’ to ensure the qualification of personnel responsible for product quality and safety.
Learn-IQ allows organizations to conduct trainings and manage personnel competency in line with global regulations with comprehensive audit trails and 21 CFR Part 11 compliant electronic signatures.
Having complete details of number of trainings scheduled, trainees attending, certifications, re-certifications of trainers happening at one glance in important. In simple words, a training dashboard would help the management to understand the training related concerns and take effective decisions. Higher-up can simply dig into the details of the specific reports with a few clicks on the dashboard.
Learn-IQ, with its drilldown dashboards, configurable reports and analytics on the go is a best bet for highly regulated industries.










USA, Pittsburgh
1(412) 499 3442
1 888 510 LIMS
sales@caliberuniversal.com
1701 McFarland Rd.,
Suite 200 Pittsburg, PA 152161812
India, Ahmedabad
91-40-30911555
91-40-23811322
91-40-30911552
sales@caliberuniversal.com
204/A, Pinnacle Business
Park Corporate Road,
Prahlad Nagar,
Ahmedabad-380015
India, Hyderabad
91-40-30911555
91-40-23811322
91-40-30911552
sales@caliberuniversal.com
Caliber House B-36,37,
Sanathnagar Industrial Estate,
Sanathnagar, Hyderabad-500018
Singapore
(65) 8869 1135
informatics@shimadzu.com.sg
Shimadzu Singapore 79 Science
Park Drive #02-01/08,
Cintech IV Singapore
Science Park I Singapore 118264
China
86 (10) 86399268
LWL Development Limited,
Room No. 1009,
Office Building A, New
World Center, No. 3,
Chongwenmenwai Street,
Dongcheng District,
Beijing, Postal Code - 100062
Bangladesh
(880) 1841334807
praptisangma@gme-group.net
GME-Bangladesh Plot # 21,
Block-Gulshan, Bangladesh,
Road # 13, Dhaka 1212,
Bangladesh
Indonesia
(62)-021-580-6882
Shimadzu – PT Ditek Jaya Kedoya
Elok Plaza Blok DA-12 Jl.
Panjang – Kebon Jeruk
Jakarta Barat 11520
Indonesia
Turkey
+902 165660050
Netherlands
Caliber Technologies BV
(+31) 629088787
Queens Tower Delflandlaan 1 1062
EA Amsterdam The
Netherlands
Sharjah UAE
NA-SA INFORMATICS FZE
+971 50 130 4543
SAIF Zone, Q1-08-97/B, P.O.
Box 513510, Sharjah,
United Arab Emirates
Dubai UAE
Emphor Science
Technology Trading
Al Quoz Industrial Area 3,
Post box 6488, Dubai UAE
Jordan
agon
Queen Rania Street,
Build 373, Amman, Jordan