Manage activities more precisely and efficiently. Control activity trails, define mapping rules, and maintain compliance with Caliber DigiLabel.

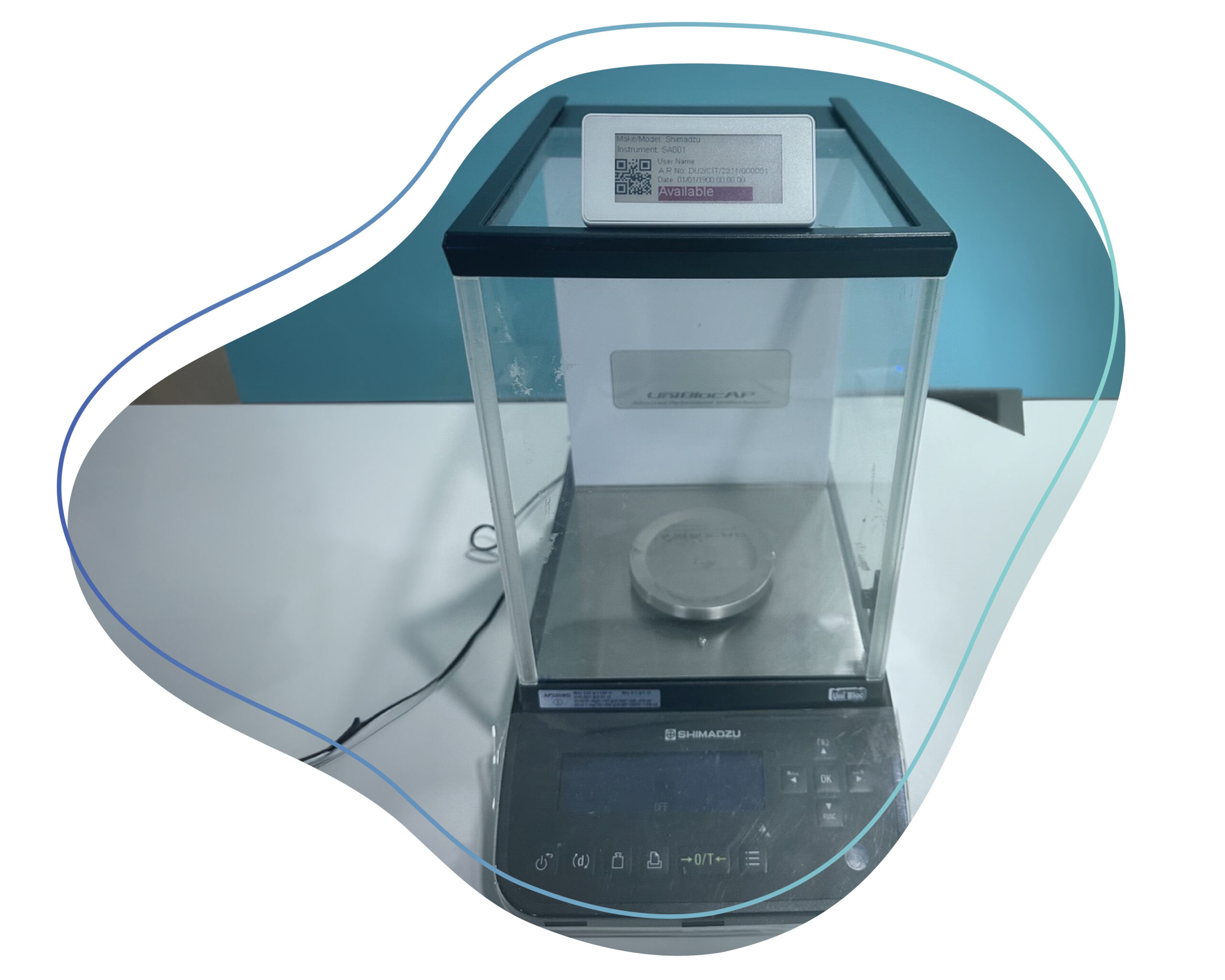
Caliber DigiLabel offers a seamlessly integrated digital ecosystem for label management. Monitor, trace, and customize labels with end-to-end digitalization. With detailed data logs, digital instrument availability status, and feature consistency across all environments, labs can ensure GxP compliance.
DigiLabel enables users to design and integrate labels seamlessly with any systems or workflows. It’s intuitive interface, configuration flexibility, and scalability lead to enhanced user-experience.
Save piles of paper label discards with changing auditor requests. Caliber DigiLabel offers an easy drag-and-drop design solution. Customize, configure, and integrate labels to tailor to specific needs easily. Quick parameter updates on labels ensure smooth lab activities and enhance lab efficiency.


Transition your labels from physical paperwork to digitalized versions, streamlining processes and increasing lab efficiency.
Ensure accurate and timely updates on instrument statuses from calibration to status labels. Monitor, trace, and customize labels effortlessly.

Monitor end-to-end logs, track changes easily, and always have updated inventory. Maintaining audit trails helps meet GLP requirements.
eDisplay integrates seamlessly. With a standard configuration/display device layer, end users can easily enable or configure the system.

Caliber eDisplay adheres to electronic labeling standards FDA 21 CFR 820. Its accurate audit trails enable faster GxP approvals increasing lab efficiency.

Our electronic label system is easy to operate and promotes seamless interaction with the systems.

Transition your labels from physical paperwork to digitalized versions, streamlining processes and increasing lab efficiency.

Ensure accurate and timely updates on instrument statuses from calibration to status labels. Monitor, trace, and customize labels effortlessly.

Monitor end-to-end logs, track changes easily, and always have updated inventory. Maintaining audit trails helps meet GLP requirements.

DigiLabel integrates seamlessly. With a standard configuration/display device layer, end users can easily enable or configure the system.

Caliber DigiLabel adheres to electronic labeling standards FDA 21 CFR 820. Its accurate audit trails enable faster GxP approvals increasing lab efficiency.

Our electronic label system is easy to operate and promotes seamless interaction with the systems.

Do not miss updates or log into the system every time for instrument availability status. DigiLabel maps data directly from source and displays updated lab activities, ensuring data integrity at all times.
USA, Pittsburgh
1(412) 499 3442
1 888 510 LIMS
sales@caliberuniversal.com
1701 McFarland Rd.,
Suite 200 Pittsburg, PA 152161812
India, Ahmedabad
91-40-30911555
91-40-23811322
91-40-30911552
sales@caliberuniversal.com
204/A, Pinnacle Business
Park Corporate Road,
Prahlad Nagar,
Ahmedabad-380015
India, Hyderabad
91-40-30911555
91-40-23811322
91-40-30911552
sales@caliberuniversal.com
Caliber House B-36,37,
Sanathnagar Industrial Estate,
Sanathnagar, Hyderabad-500018
Singapore
(65) 8869 1135
informatics@shimadzu.com.sg
Shimadzu Singapore 79 Science
Park Drive #02-01/08,
Cintech IV Singapore
Science Park I Singapore 118264
China
86 (10) 86399268
LWL Development Limited,
Room No. 1009,
Office Building A, New
World Center, No. 3,
Chongwenmenwai Street,
Dongcheng District,
Beijing, Postal Code - 100062
Bangladesh
(880) 1841334807
praptisangma@gme-group.net
GME-Bangladesh Plot # 21,
Block-Gulshan, Bangladesh,
Road # 13, Dhaka 1212,
Bangladesh
Indonesia
(62)-021-580-6882
Shimadzu – PT Ditek Jaya Kedoya
Elok Plaza Blok DA-12 Jl.
Panjang – Kebon Jeruk
Jakarta Barat 11520
Indonesia
Turkey
+902 165660050
Netherlands
Caliber Technologies BV
(+31) 629088787
Queens Tower Delflandlaan 1 1062
EA Amsterdam The
Netherlands
Sharjah UAE
NA-SA INFORMATICS FZE
+971 50 130 4543
SAIF Zone, Q1-08-97/B, P.O.
Box 513510, Sharjah,
United Arab Emirates
Dubai UAE
Emphor Science
Technology Trading
Al Quoz Industrial Area 3,
Post box 6488, Dubai UAE
Jordan
agon
Queen Rania Street,
Build 373, Amman, Jordan