Transforming electronic batch records for the future

Advanced manufacturing digitalization
CaliberBRM is a value-added electronic batch record software that ensures a fully compliant, smooth transition to seamless manufacturing digitalization.
With traceable, easy-to-comprehend batch records, automated logbooks, and consistent in-process quality checks, CaliberBRM offers advanced digitalization for manufacturing enterprises. Its light-weight architecture is flexible, accessible, and scalable beyond manufacturing execution systems (MES) capabilities for batch manufacturing.
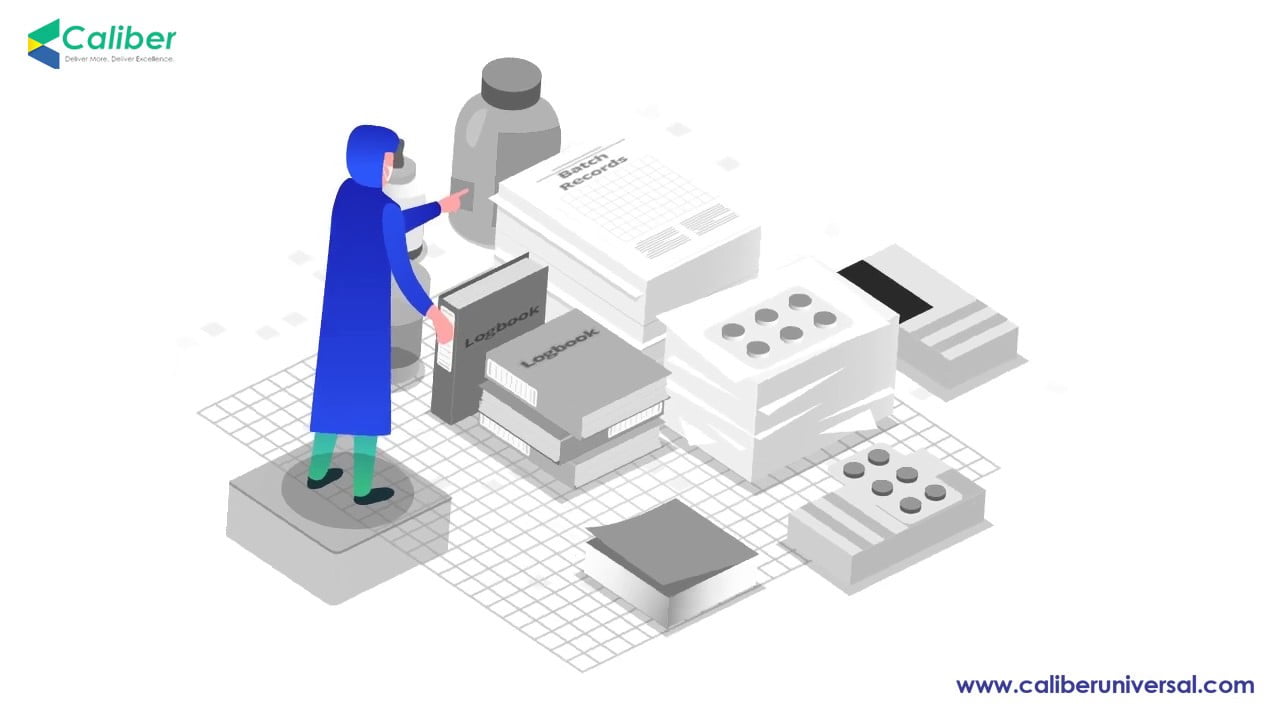
Electronic batch record management software at its best!
100% paperless manufacturing is now possible with a complete digital batch record management system.
End-to-end paperless manufacturing solution
CaliberBRM eases rigid manufacturing frameworks. Configurable templates, flexible workflows, seamless integrations, and easy implementation ensure a smooth, strategic transition to paperless manufacturing.
Our advanced eBRM software accelerates batch releases with timely alerts for discrepancies, thus eliminating errors consistently. With CaliberBRM scalability is always compliantly possible.
Feature-rich, stand-alone modules enrich our digital manufacturing software
Automate batch records for accelerated quality management, enhanced data integrity, and error-free manufacturing. With accurate batch record management and streamlined workflows, businesses achieve consistent and sustainable operational efficiency.
CaliberBRM promotes electronic batch records that are reliable, traceable, and easily retrievable. Eliminating the most common cause of FDA warning letters, our EBR module ensures that batch records are appropriately documented and presented for audit inspections.
Manage your batch records efficiently with traceable, fully connected, digitized records.
CalibereLog eliminates the hassle caused by piles of paper logs, misplaced logbooks, and untraceable handwritten entries. Digitizing logs help you capture, track, and maintain logs in real-time. Get secure, compliant data always with our electronic log management software.
Reduce human errors, data security, and compliance issues with paperless llogbooks. Automating batch manufacturing logbooks ensure smooth execution of production processes.
Electronic logs provide instant access to time-stamped records and ensure audit-readiness always.
Gain complete control of product quality with consistent, automated, digitalized in-process quality checks. CaliberIPQC monitors, controls, and improves manufacturing efficiency at every stage.
Assess product quality and streamline manufacturing processes without missing samples to ensure quality drug production. Consistent checks, scheduled alerts, and timely notification of discrepancies maintain compliance with specifications.
CaliberIPQC is an independent module within CaliberLIMS that keeps a check on adherence to quality-specific manufacturing requirements.
Monitor punches and dies, manage inventory, automate inspection, and discard rules exclusively with the exclusive Punches and Dies module of CaliberBRM. Automated management of punches and dies ensures the efficacy of tool heads, concentration of dies and maintains inventory.
A unique offering under our robust batch manufacturing software solution, Punches and Dies module dedicatedly monitors the life cycle, establishes SOPs, and triggers alerts when replacement is needed.
With CaliberBRM, manufacturers are empowered with skillful management and strict control of punches and dies.



Customer Success Story
Zydus, a global manufacturer of generic drugs and other pharmaceutical formulations faced challenges in sample analysis and in-process quality checks.
Manual systems often led to missing sample analysis schedules and error-prone in-process quality checks that also risked data integrity. In this case study, Zydus shares how digitalizing with CaliberIPQC helped them to reduce the duplication of tasks and deviations. The figures prove exceptional digital transformation and success at Zydus.
Learn more about their journey in overcoming everyday challenges and how they elevated the quality standards of its manufacturing processes with CaliberIPQC.
Go Paperless Conveniently & Compliantly
CaliberBRM is a 360° framework that incorporates complete management of the batch manufacturing process and ensures that the batch records are retrievable, trackable, and error-free.
The robust batch record manufacturing solution is designed to build, track, and execute quality manufacturing processes with ease. Enabling paper-free, 21 CFR Part 11-compliant manufacturing, the software digitalizes and accelerates the entire production cycle.
- Seamless transition
- Stage-wise implementation
- Anytime audit-readiness

Strategize your digitalization roadmap today
Register for the workshop to understand your Shopfloor Maturity and plan a smooth digital transformation.
BRM Feature List

Right First Time Approach
Quality essentials in place promotes well-connected systems, automated data collection for error-free production.

Review by Exception
Reduced review time accelerates batch release and enhances profitability.

Controlled EBRs
Controlled execution of electronic batch records leads to effective batch production.

Customized BMRs
Easily configurable and reusable templates allow creation of customized BMRs.
Configurable Reporting
Configurable reports enable advanced data analysis and foster informed business decisions.

Trend Analysis
Readily accessible trend highlights for different stages of critical process parameters.