Say goodbye to paper documentation risks and inefficiencies. Docs-IQ, the web-based document management system, eliminates compliance issues and streamlines processes and ensures alignment with SOPs, GMP, and regulatory guidelines effortlessly.
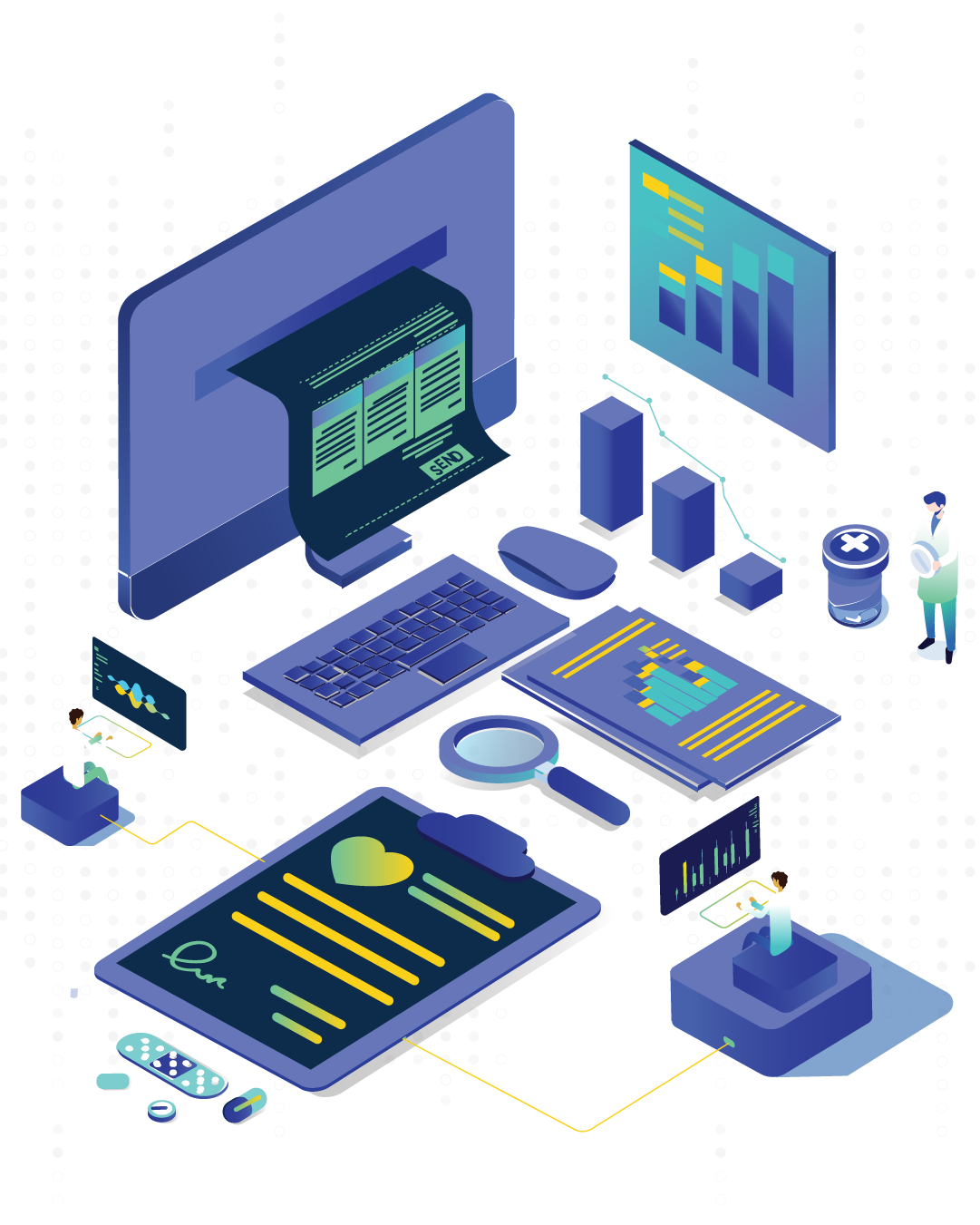
Pharmaceutical industries deal with diverse types of documents daily. In no time, the pharma companies will be drowned in paper documents if they maintain every little detail in paper. Having a simplified system for document processes is the need of the hour.

A document goes through 9 stages before it becomes obsolete or is destroyed. Management of document lifecycle from the initiation stage to storage and finally destruction in a manual paper-based company is difficult. At every stage of document lifecycle, a lot of paperwork, time, effort, and resources get involved.
You can easily control and take charge of the complete documentation lifecycle by using a dynamic document control software like Docs-IQ. The 9 stages of document lifecycle are fully automated leaving no room for transcriptional errors, back-dated entries, direct changes in SOPs, etc.
Tons of paper logbooks are bought and used every year for all types of logbooks during production. Many people are involved in recording entries and maintenance of record books. A huge amount is spent as storage cost, conveyance charges for for fetching logbooks from storage during audit. Altogether, paper-based documentation is an expensive affair.
Automation of documents is a one-time expense that helps in reducing the direct and indirect operational costs every year. It a win-win situation for companies using Docs-IQ with no paper, personnel, storage, conveyance, and other costs.

Utter chaos occurs if there are no proper guidelines for documentation of production process.
In life sciences and other regulated industries documentation is performed following stringent guidelines. Every regulated industry must be compliant to cGMP, ALCOA+, FDA, MHRA, ISO, and many such regulatory bodies’ documentation guidelines. With Docs-IQ, a comprehensive document management solution, you will be able to achieve compliance and data integrity.

Track all the edits and the revisions made in a document. See full information of the changes, users, timestamp, and preserve the original document with version control.
Align the documentation tasks by easily configuring document workflows. Automate and manage the entire document control process by creating custom flows.
Reduce the document cycle process with timely prompts on approvals, revisions with automated routing and escalation. Get prompts for the next tasks and be proactive.

Easily communicate and assign tasks for reviews and approvals across teams and departments.

Automatic system updates and tracking of issuance and retrieval of documents like BPR, BMR in the required formats.

Do quick at-a-glance reviews by adding and viewing comments on sticky notes. Make swift changes in the document without the actual edits.

Track all the edits and the revisions made in a document. See full information of the changes, users, timestamp, and preserve the original document with version control.

Align the documentation tasks by easily configuring document workflows. Automate and manage the entire document control process by creating custom flows.

Reduce the document cycle process with timely prompts on approvals, revisions with automated routing and escalation. Get prompts for the next tasks and be proactive.

Easily communicate and assign tasks for reviews and approvals across teams and departments.

Automatic system updates and tracking of issuance and retrieval of documents like BPR, BMR in the required formats.

Do quick at-a-glance reviews by adding and viewing comments on sticky notes. Make swift changes in the document without the actual edits.
Sometimes a change or deviation may trigger a change in SOP. In manual paper-based process, it would take much time for the tracking the document, making the changes, completing the review and approval process, training the QA, etc. It is imperative to choose a document management system that can integrate with other applications, ERP systems to pull data automatically and avoid manual intervention.
With Docs-IQ and its seamless integration with quality systems and any ERP system, you can trigger a deviation or change request in QMS to make changes in the SOPs directly in the DMS and schedule trainings in CMS. That simple!








USA, Pittsburgh
1(412) 499 3442
1 888 510 LIMS
sales@caliberuniversal.com
1701 McFarland Rd.,
Suite 200 Pittsburg, PA 152161812
India, Ahmedabad
91-40-30911555
91-40-23811322
91-40-30911552
sales@caliberuniversal.com
204/A, Pinnacle Business
Park Corporate Road,
Prahlad Nagar,
Ahmedabad-380015
India, Hyderabad
91-40-30911555
91-40-23811322
91-40-30911552
sales@caliberuniversal.com
Caliber House B-36,37,
Sanathnagar Industrial Estate,
Sanathnagar, Hyderabad-500018
Singapore
(65) 8869 1135
informatics@shimadzu.com.sg
Shimadzu Singapore 79 Science
Park Drive #02-01/08,
Cintech IV Singapore
Science Park I Singapore 118264
China
86 (10) 86399268
LWL Development Limited,
Room No. 1009,
Office Building A, New
World Center, No. 3,
Chongwenmenwai Street,
Dongcheng District,
Beijing, Postal Code - 100062
Bangladesh
(880) 1841334807
praptisangma@gme-group.net
GME-Bangladesh Plot # 21,
Block-Gulshan, Bangladesh,
Road # 13, Dhaka 1212,
Bangladesh
Indonesia
(62)-021-580-6882
Shimadzu – PT Ditek Jaya Kedoya
Elok Plaza Blok DA-12 Jl.
Panjang – Kebon Jeruk
Jakarta Barat 11520
Indonesia
Turkey
+902 165660050
Netherlands
Caliber Technologies BV
(+31) 629088787
Queens Tower Delflandlaan 1 1062
EA Amsterdam The
Netherlands
Sharjah UAE
NA-SA INFORMATICS FZE
+971 50 130 4543
SAIF Zone, Q1-08-97/B, P.O.
Box 513510, Sharjah,
United Arab Emirates
Dubai UAE
Emphor Science
Technology Trading
Al Quoz Industrial Area 3,
Post box 6488, Dubai UAE
Jordan
agon
Queen Rania Street,
Build 373, Amman, Jordan