Revolutionizing Labs
for a Better Tomorrow
For over twenty years, CaliberLIMS has been a vital asset to labs worldwide. With a feature-rich and fully compliant solution, CaliberLIMS turns the paperless lab dream into reality.

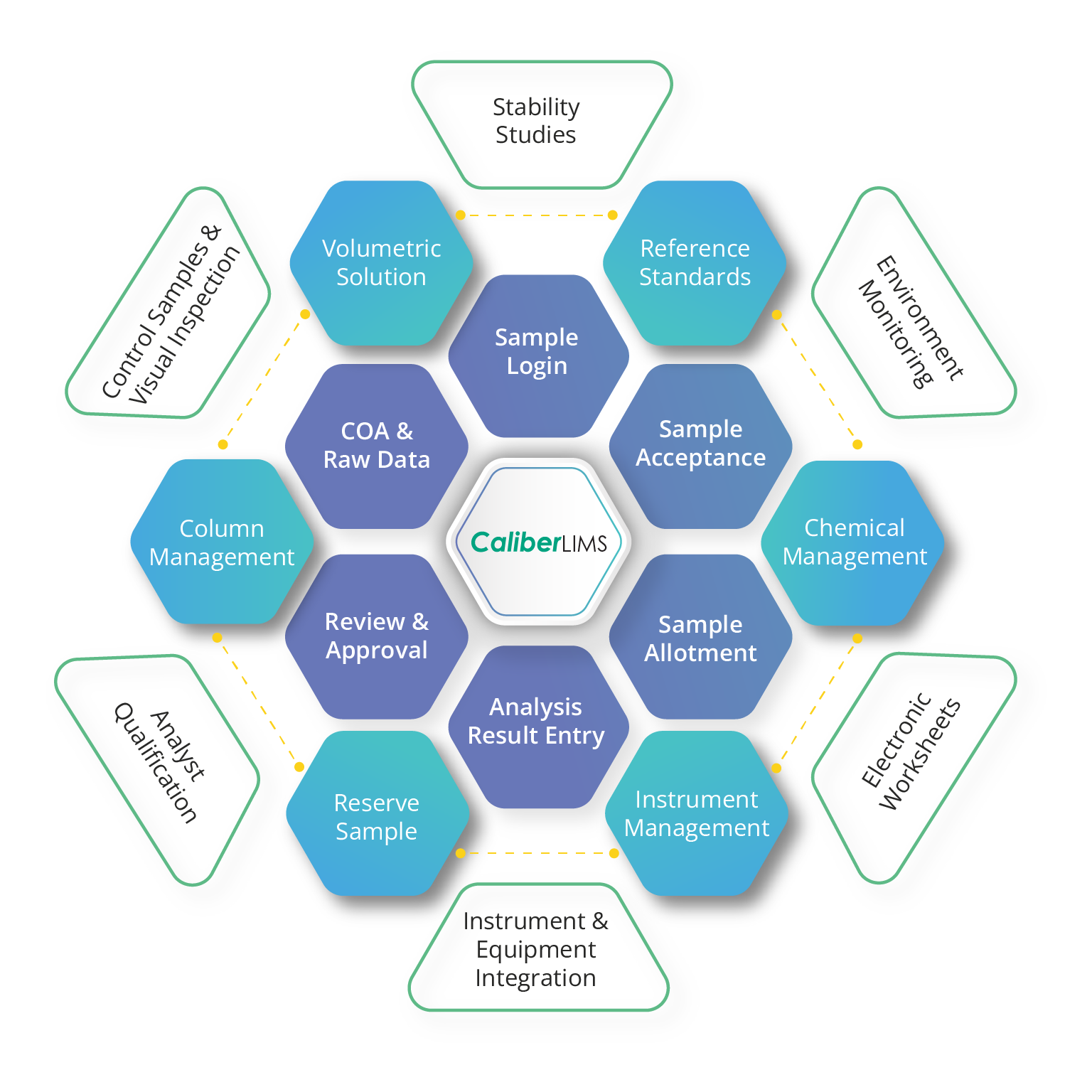
Intrinsically Designed to Accommodate Change
CaliberLIMS offers 16+ robust modules and flexible features that can fit labs of all sizes. Unique features and seamless configurations help labs realize their dream of 100% paperless operations.
Easing integration with emerging technologies and fast-paced labs, CaliberLIMS is a one-fit laboratory solution for future labs.

Customer
Success Story
MSN Laboratories transformed their lab operations with CaliberLIMS. To enhance lab processes and efficiency, MSN decided to take the paperless route. CaliberLIMS was implemented across the formulation units which helped MSN achieve a zero-paper lab.
- Improved process efficiency
- Increased data integrity
- Successful, worry-free audits

Seamless Lab Integration
Seamless integration with ERP systems, QMS, DMS, CMS, CDS, file, and port-based instruments for data integrity.

Track with Built-in ELN
With a built-in ELN (electronic laboratory notebook), keep a track of all the lab activities effortlessly.

Meet Compliance Standards
Satisfies FDA’s 21 CFR part 11, EU Annexure 11, GMP, and other regulatory requirements where all transactions are audit trailed.

Make Smart Decisions
Instant analytics and trends generated from the information available in the system make smart decision-making possible.
Simplified & Accurate Stability Testing
Streamline and accelerate stability studies with a robust stability module within CaliberLIMS. An automated stability testing process monitors drug and product shelf life, safety, and efficacy.
Ensure data consistency, get faster access to test results, and improve collaboration with a centralized stability management system. Lab analysts can pre-define stability protocols, track samples, manage software integrations, and maintain data integrity as per regulatory requirements.


End-to-End Sample Management
Managing samples efficiently is the first step to obtaining operational laboratory excellence. CaliberLIMS automates the entire sample lifecycle. Lab managers and analysts serve the bigger purpose and become more agile with an efficient Sample Management Software in place. With standardized workflows, labs can be confident of increased efficiency, accelerated sample release, and enhanced data integrity.
Touching all the phases of a sample’s lifecycle once it enters the laboratory, CaliberLIMS gives users a complete view of the happenings, allows them to address loopholes, presents a detailed analysis of sample trends, and informs of gaps to be addressed.
Game-changing Glassware
Scannable glassware in laboratories? Digitalized laboratories must have them. CaliberLIMS is a pioneer in partnering with Glassco’s QR-LabGlass Smart Flask with a uniquely etched QR code. Instruments come to life with premium quality material with lifetime embossed identity. Glassware can be tracked and managed with just a single scan.
The Glassware Module empowers labs with:
- Easy identification and classification
- Smart inventory management
- Enhances traceability and usage
- Ensured compliance
- Prevention of incorrect usage
Glassware traceability is essential to understand the movement of instruments within labs.



Start your lab’s next-gen digital transformation today
Prepare your labs for the uncertain future while also being resilient and compliant. It is imperative to reinvest in digitalization for the changing regulatory environment, quality-driven operations, and future readiness.
Discover how to build tomorrow ready labs
- Essential factors to build a resilient lab
- 3 key themes that influence today’s digital decisions
- Practical approach to efficient, 100% paperless labs
OOT Configuration
OOT (Out Of Trend) configuration master will provide an option to set the OOT limits for the respective tests under the selected specification. An option to set the OOT limits using the 3 sigma or Custom Range. Stability initial sample and previous station comparison.

Dossier generation
Dossier will provide an option to generate the batch-wise e-booklet with all necessary details by collecting the samples from the Sample Login till the Batch release (COA, Raw data, Instrument reports, Vendor reports, etc.,). Users will have the option to select the required set of documents to submit the Dossier request and generate the document with password protection.
Analyst qualification
Analyst Qualification Module covers both Complex and Non-Complex Techniques. Complex techniques will be evaluated with the execution of the released batch with additional A.Q. limits. Whereas Non-Complex techniques will be evaluated based on discussion and Q&A methodology.

Reserve Sample Management
Reserve Sample module enables reserve sample inventory with sample details and quantity. Samples can be drawn from the inventory in case of any need. The System keeps track of the quantity withdrawn, quantity returned, and purpose of sample withdrawal. Samples can be marked for destruction based on the period or through selection. Also, Reserve Sample can be marked for the visual inspection as per the defined frequency and checklist. Reports include reserve sample inventory and usage records.
Smarter labs with limitless integrations
A perfect lab scenario is realizable. With CaliberLIMS, labs can be fully connected, integrating seamlessly with systems and instruments. Prioritize lab integrations to achieve a productive, compliant, and successful lab. Increase efficiency, streamline production processes, and amplify operations with a structured approach.
Glassware Management
Glassware module facilitates information on various Glassware used in the lab and the related activities. The Glassware Management Module covers the activities like registration of Glassware Type, Glassware Lot, Lot Acceptance, Issuance, Usage, and generation of Reports. Glassware QR code tracking is an added advantage for easy tracking and usage.

Adcock Ingram Enhances Quality and Meets Compliance with Paperless Lab
Download the case study to witness their digital journey that enhanced their processes and assisted them achieve quality.