Manage activities more precisely and efficiently. Control activity trails, define mapping rules, and maintain compliance with CaliberDigiLabel.

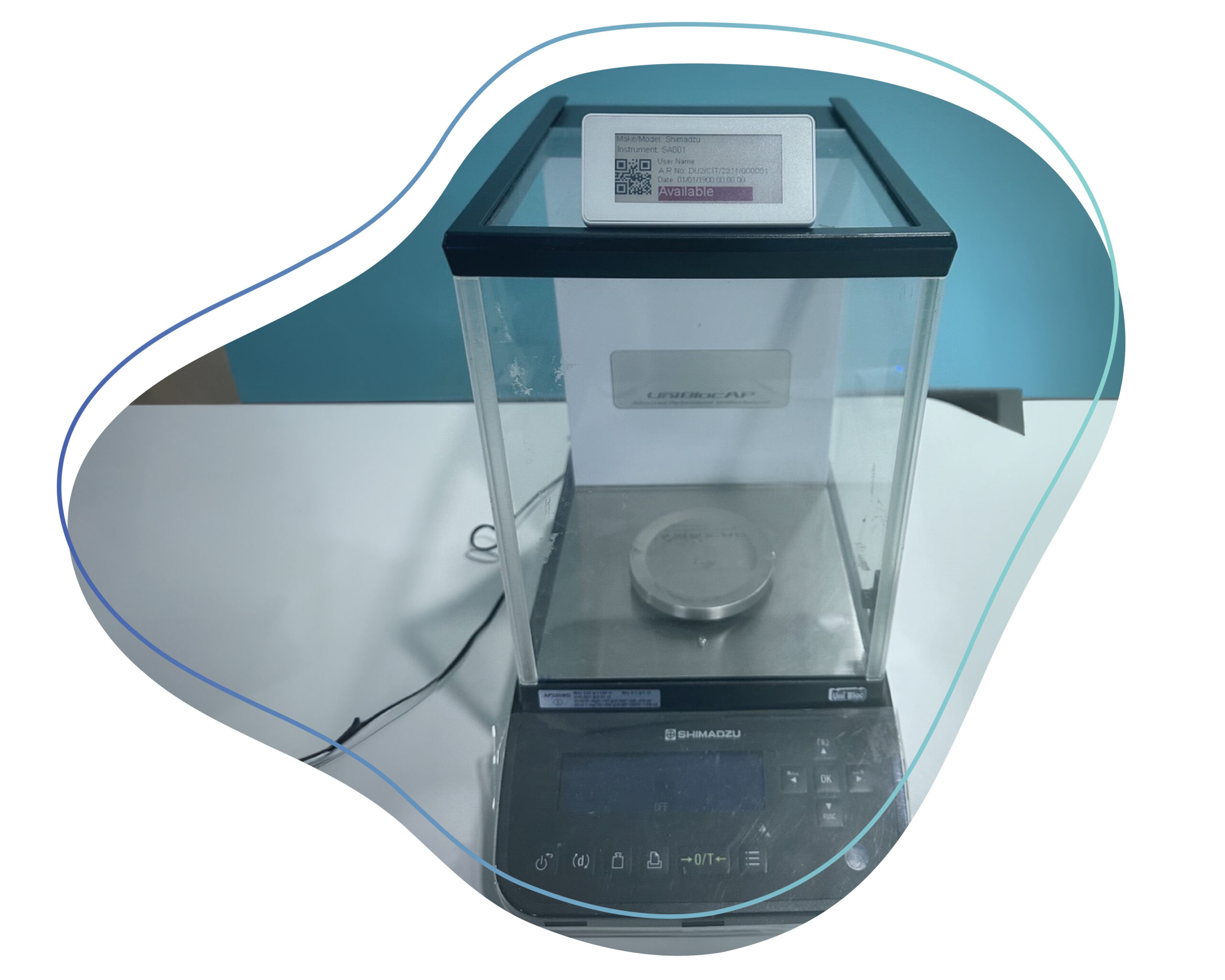
CaliberDigiLabel offers a seamlessly integrated digital ecosystem for label management. Monitor, trace, and customize labels with end-to-end digitalization. With detailed data logs, digital instrument availability status, and feature consistency across all environments, labs can ensure GxP compliance.
It enables users to design and integrate labels seamlessly with any systems or workflows. It’s intuitive interface, configuration flexibility, and scalability lead to enhanced user-experience.
Save piles of paper label discards with changing auditor requests. CaliberDigiLabel offers an easy drag-and-drop design solution. Customize, configure, and integrate labels to tailor to specific needs easily. Quick parameter updates on labels ensure smooth lab activities and enhance lab efficiency.


Transition your labels from physical paperwork to digitalized versions, streamlining processes and increasing lab efficiency.
Ensure accurate and timely updates on instrument statuses from calibration to status labels. Monitor, trace, and customize labels effortlessly.

Monitor end-to-end logs, track changes easily, and always have updated inventory. Maintaining audit trails helps meet GLP requirements.
eDisplay integrates seamlessly. With a standard configuration/display device layer, end users can easily enable or configure the system.

Caliber eDisplay adheres to electronic labeling standards FDA 21 CFR 820. Its accurate audit trails enable faster GxP approvals increasing lab efficiency.

Our electronic label system is easy to operate and promotes seamless interaction with the systems.

Transition your labels from physical paperwork to digitalized versions, streamlining processes and increasing lab efficiency.

Ensure accurate and timely updates on instrument statuses from calibration to status labels. Monitor, trace, and customize labels effortlessly.

Monitor end-to-end logs, track changes easily, and always have updated inventory. Maintaining audit trails helps meet GLP requirements.

CaliberDigiLabel integrates seamlessly. With a standard configuration/display device layer, end users can easily enable or configure the system.

CaliberDigiLabel adheres to electronic labeling standards FDA 21 CFR 820. Its accurate audit trails enable faster GxP approvals increasing lab efficiency.

Our electronic label system is easy to operate and promotes seamless interaction with the systems.

Do not miss updates or log into the system every time for instrument availability status. CaliberDigiLabel maps data directly from source and displays updated lab activities, ensuring data integrity at all times.