





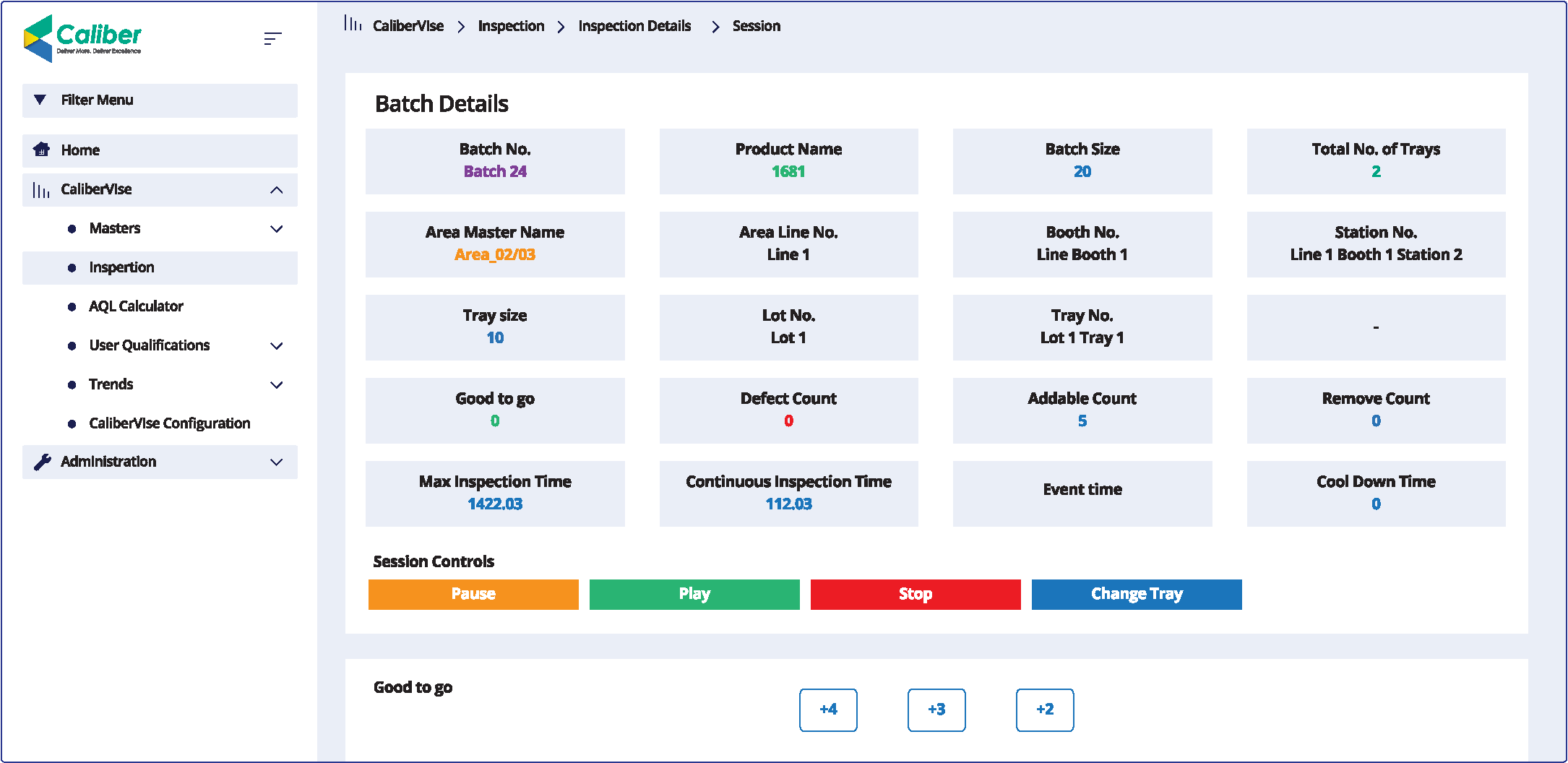
CaliberVIse helps quality inspectors reduce fatigue-driven errors, automate AQL decisions, and deliver complete GMP traceability across every vial, shift, and inspector. It transforms manual vial inspection into a controlled, compliant, and traceable quality process-ready for Pharma 4.0.

Traditional visual inspection relies heavily on human effort, manual counting, time tracking, defect logging, and post-inspection reconciliation.
CaliberVIse replaces disconnected tools with a single, digitally governed inspection workflow. It actively controls inspection time, enforces rest intervals, standardizes defect classification, and captures real-time data, ensuring consistency, traceability, and compliance by design.

In conventional setups, inspection data is reviewed after the fact, delaying AQL decisions, investigations, and batch disposition. This reactive approach limits visibility into emerging trends and increases deviation and recall risk.
CaliberVIse delivers real-time dashboards, automated AQL calculations, and instant alerts when thresholds are crossed. Supervisors and QA teams gain immediate insight into inspection performance, defect trends, and batch status—enabling faster, confident, and defensible decisions.
CaliberVIse is purpose-built for human-led visual inspection in regulated pharmaceutical environments. Rather than replacing inspectors, it strengthens human performance through digital controls that align with GMP, FDA, and data integrity expectations.
With built-in session management, fatigue controls, inspector qualification, and 21 CFR Part 11–compliant records, it embeds compliance directly into daily inspection operations, reducing manual effort while increasing regulatory confidence.

Structured, real-time inspection workflows that reduce manual effort and improve focus.
Enforced inspection times, automated breaks, session tracking, and full shift continuity.
Automated AQL management, defect classification, tamper-evident records, and audit-ready reports.

Embedded inspector qualification, live training assessments, re-qualification scheduling, and access control.
Faster batch decisions, improved productivity, reduced fatigue errors, stronger audit readiness, and lower compliance risk.
Help teams move beyond routine checks. Converts inspection activity into a data-rich, insight-driven quality process




