An Integrated Approach to Enterprise Quality
EPIQ QMS by Caliber is a complete quality management system that centralizes quality, enhances compliance, and controls quality risk. EPIQ beautifully connects quality events, documents, and learning into a single powerful, scalable, and reliable QMS.
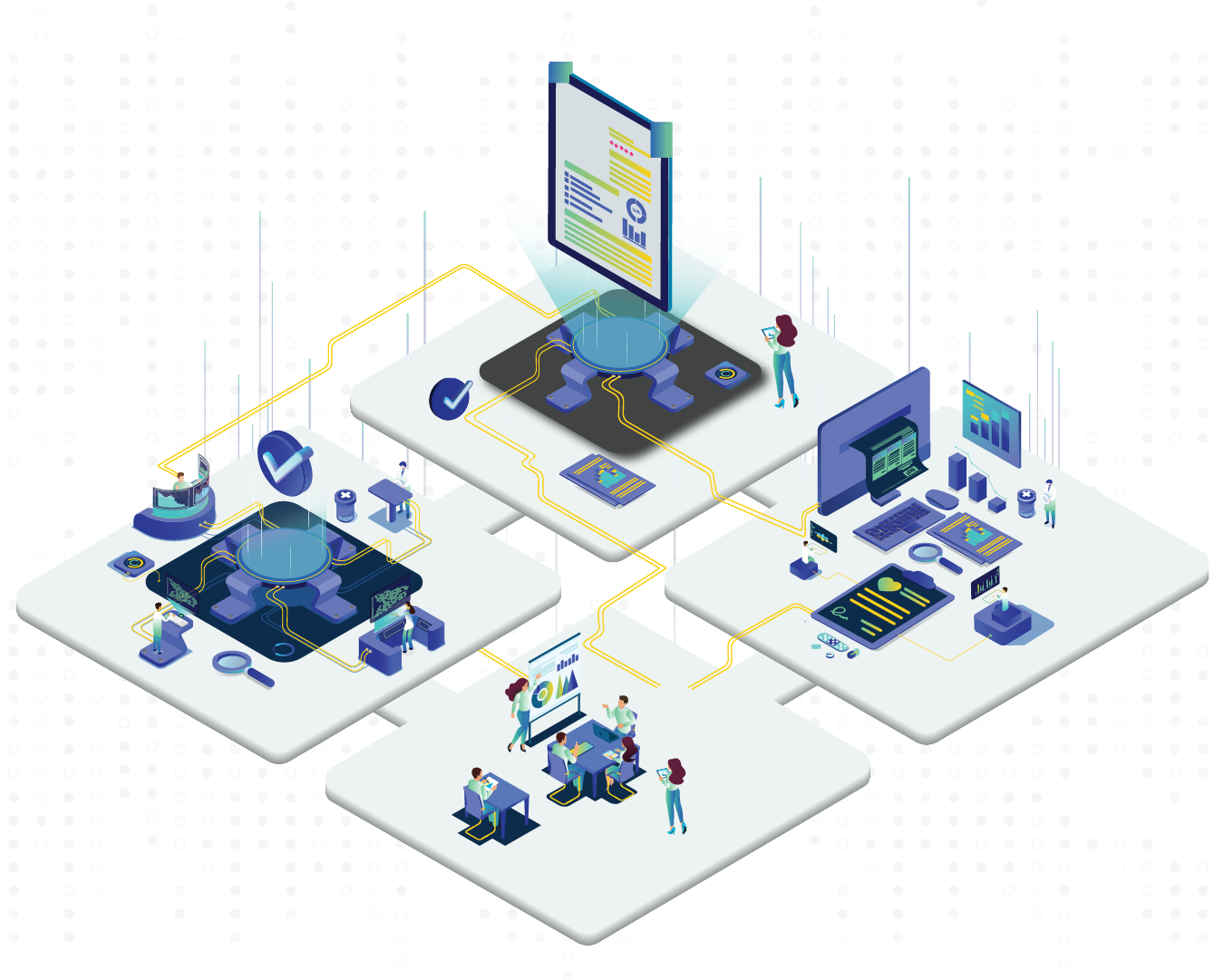
A fully automated quality management software by Caliber.
A digital QMS maintains data accuracy and consistency effortlessly. assure-iq accelerates approval processes, thus preventing costly delays. Accurate, consistent data throughout facilitates easy-to-access audit trails and document history.
Achieve 100% data integrity, audit compliance, and quality excellence. Configurable workflows and business rules simplify the quality assurance process. Our robust quality management system blends and integrates into your company’s systems easily. Assure-iq by Caliber is reliable, trusted, and fully featured.
A Complete Digital Document Control Management Software
Eliminate stacks of paper documents with Docs-iq by Caliber. Save space, risk, and effort to gain operational efficiency. With paperless documentation, maintaining and tracking document version history becomes easy. Docs-iq ensures real-time collaboration with quality systems and facilitates precise change control mechanism.
Digitalized document management software simplifies document access, retrieval, and maintenance. Docs-iq by Caliber maintains data integrity, regulatory compliance, and reduces storage costs.
A Robust Learning Management Software by Caliber
Learn-iq is an automated, highly efficient learning management software. Managing training activities as per regulatory requirements ensures error-free, complete audit trails. Monitoring control over learning curves – employee assessment, training, and result mapping, Learn-iq by Caliber promotes role-based learning and auditory compliance.
With an automated learning management system, get only qualified personnel to perform critical tasks. Enable simple, traceable training workflows for a confident, competent, and continuously improving workforce with Learn-iq.

EPIQ Overview Video
Automating quality throughout the product lifecycle, EPIQ by Caliber ensures end-to-end digital quality assurance. Caliber follows an integrated quality approach with EPIQ QMS – futuristic, quality-centric, fully compliant software.


Resonating The Power of 3
Streamlined integration between quality systems ensures a closed-loop, highly compliant and efficient quality success.
Epiq by Caliber is the perfect demonstration of a closely-knit quality structure for highly regulated industries. Triggering relevant change control mechanisms automatically, the integrated quality management software ensures compliance, mitigates risks, and controls quality execution at all levels.
- A change control raised in assure-iq needing a document creation or modification: Workflow triggered for assure-iq travels to docs-iq. docs-iq manages the document lifecycle. On completion of document creation or modification process in docs-iq, the workflow enables closure of change control in assure-iq.
- assure-iq can be integrated with any third-party document management system.
- Training requirements identified before publishing a document: The workflow travels from docs-iq to learn-iq to ensure the process of training the people in various departments. Once the training is marked complete the workflow travels back to docs-iq and enables publishing of the document.
- Training requirement gets automatically initiated due to the re-issuance of a document either due to modifications or revisions.
- Change control raised in assure-iq for SOP which mandates training of people will enable the workflow to be moved to learn-iq for the next steps.
We have empowered 250+ businesses with EPIQ QMS – our centralized quality system for future-ready, resilient enterprises.
Why Integrated Quality Matters
EPIQ by Caliber resonates with the power of 3 systems – Quality Management, Document Management, and Learning Management. Easy access to quality data maintains integrity, drives business growth, and increases operational efficiency.

Quality Management Whitepaper
Inter-departmental information exchange while adhering to the quality metrics is a practical challenge. A QMS that caters to the 360° integrated quality requirements of an enterprise is now available.
Get your hands on a unified QMS platform that is powerful, highly customizable, ready-to-use, and scalable.
Get insights on:
- The role of QMS in operational excellence
- ROI with an integrated quality management solution
- Three-dimensional benefits of the enterprise-wide quality platform
- Sample use cases

Seamless Quality Management
Promote a smooth flow of quality across the enterprise – effortlessly and compliantly.

Industry Independent
Enterprise-wide quality infrastructure for all, irrespective of industry size and type.
SaaS Availability
Pay-as-you-go cloud availability for strategic digital transformations.
Compliant CAPA Management
Enable continuous quality improvement for reduced product recalls.
Power of 3
Overcome the hassle of managing different windows. Single sign-on administrator window for QMS, DMS & LMS.
Integrated Reporting
Improve quality and process management across functions with insightful trends and analysis.
Epiq SaaS model
Explore the next phase of quality modernization. EPIQ QMS allows on-premise and cloud hosting for convenient and strategic digital transformation. It is swift, easy, and compatible.
Now, you can also choose to pay for a specific feature set. With SaaS availability, EPIQ QMS leverages the potential to gear up quality at a flexible pace. We help implement quality effectively across your organization, the way you want.
Integrated Quality Matters
With quality ensured at every stage, EPIQ QMS weaves it into every action that matters, creating a highly vigilant, traceable, and efficient manufacturing ecosystem. Quality and compliance are easily achievable goals with EPIQ QMS.
Bringing industry’s best quality practices on a central platform, epiq by Caliber offers the flexibility to meet every business’s individual needs. Leverage quality excellence with one of the finest eQMS – epiq by Caliber.