What’s Inside
ToggleThe pressure to meet FDA quality requirements is an inevitable state. This summer, manufacturers will feel the heat with strict regulatory scrutiny and the growing demand for quality metrics to improve operational efficiency. Though a modern-day approach calls for a rapid transition from reactive compliance practices to proactive, data-driven POV’s, the changing pharma environment asks for a little more. A risk-based quality management approach.
Wonder why even the smartest pharma manufacturers relied on reactive systems for quality metrics for decades. These systems were widely popular for their delayed response to quality issues; they were often too late to prevent the risks and costly product recalls.
Compliance Requirements by the FDA
FDA compliance and quality metrics requirements present pharmaceutical manufacturers with a dual challenge: maintaining rigorous FDA compliance and ensuring product quality that meets the highest of the FDA standards, as highlighted in the FDA’s intent to implement a modern, risk-based pharmaceutical quality assessment system.
In disguise, this development offers immense opportunities for pharmaceutical manufacturers. Real-time access to critical data is not just a luxury; now it’s a necessity for sustained compliance, improved operational efficiency, and enhanced product quality.
FDA Quality Metrics: What Matters the Most?
The FDA’s Quality Metrics Initiative, which emphasizes real-time data, transparency, and continuous monitoring, is one characteristic of robust site Quality Management Maturity (QMM). The change has significantly reshaped the regulatory landscape. FDA now expects manufacturers to adopt a more proactive approach, using data to anticipate and address potential risks before they manifest. Quality metrics are used throughout the drugs and biologics industry to monitor quality control systems and processes and drive continuous improvement efforts in drug manufacturing.
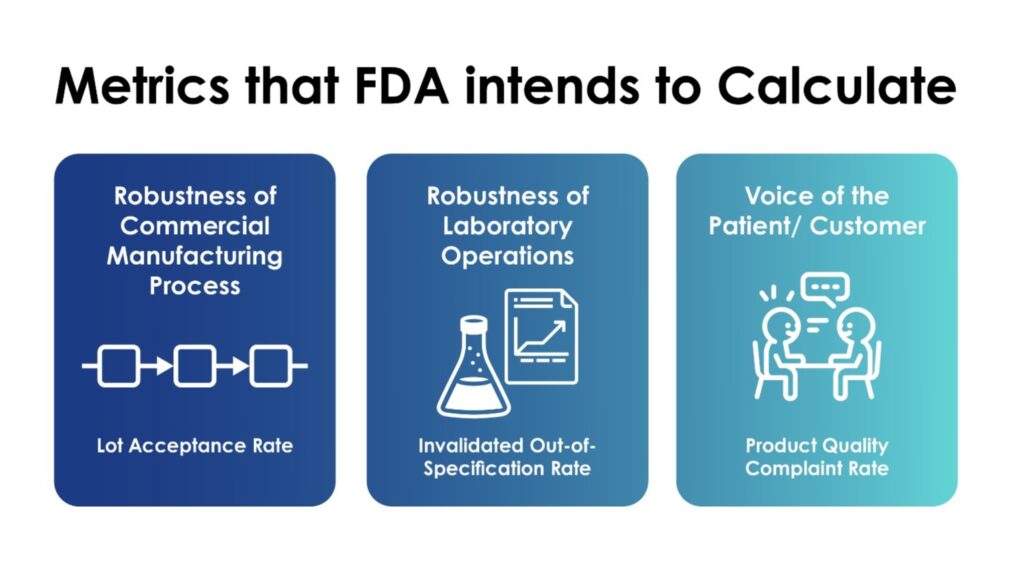
These metrics can also be useful to the FDA:
- To help develop compliance and inspection policies and practices, such as risk-based inspection scheduling for drug manufacturers.
- To improve the agency’s ability to predict and, therefore, possibly mitigate future drug shortages.
- To encourage the pharmaceutical industry to implement state-of-the-art, innovative quality management systems for pharmaceutical manufacturing.
The FDA Quality Metrics initiative aims to ensure that pharmaceutical companies provide safe, effective products by promoting quality management practices based on actual, real-time performance data.
‘The Food and Drug Administration Safety and Innovation Act (FDASIA) of 2012 provides the FDA with new authorities to ensure the safety, quality, and availability of the drug supply chain’
One section of FDASIA calls for the FDA to replace the current biennial inspection of all pharmaceutical manufacturing sites with a risk-based inspection program and provides the FDA with the authority to obtain information in advance of inspection, i.e., company-supplied metrics, to support such a program. “An objective set of quality metrics” would be reportable to support their risk-based inspection program, choosing standardized data and metrics that would be reported. Some of this information is quality metrics data, which includes key quality metrics that reflect the health of a manufacturing process. _ FDA
How Can Proactive Compliance Be Achieved?
The proactive approach is for many pharmaceutical manufacturers that have historically relied on manual data entry, lagging indicators, and periodic reporting. Methods that often fail to provide the timely insights needed to mitigate risks.
The future of pharmaceutical manufacturing is digital, and Industry 4.0 is much more than just digitization. It’s about leveraging real-time data to drive continuous improvement, improve decision-making, and ensure FDA compliance. As manufacturers move toward more agile and data-driven operations, getting the key quality metrics in real-time becomes even more integral to their success.
How CaliberMetrix is Transforming FDA Compliance and Quality Management
CaliberMetrix is a pharma-focused solution that helps pharmaceutical manufacturers move from a time-based to a risk-based, audit-ready approach. It offers real-time access to key quality metrics to allow pharmaceutical manufacturers to continuously monitor their processes, identify potential risks early, and streamline compliance reporting. Real-time quality insights also allow manufacturers to make faster, more informed decisions, preventing issues from escalating.
Download CaliberMetrix brochure to learn more about the key quality metrics that matter the most to the FDA.
Impact of CaliberMetrix on Quality and Compliance
The innovative quality metrics solution leads a smooth transition toward Industry 4.0, which involves the integration of digital tools, automated systems, and real-time data analytics, and highlights the essentials for driving proactive compliance.
- Continuous Monitoring Equals Continuous Improvement
Real-time data and instant feedback enable manufacturers to track their processes in minutes, so they can find areas of improvement and fix quality problems before they become major issues. This promotes a culture of ongoing quality improvement, where manufacturers are continually reaching for better.
- Streamlining Compliance
Automated compliance reporting, real-time access to metrics, and an integrated system reduce manual errors. The platform streamlines the compliance process, making it easier for manufacturers to maintain regulatory adherence.
- Building FDA Trust
The platform’s ability to maintain detailed, accurate records builds trust with the FDA. Pharma manufacturers can be confident at the time of audit and show that they are committed to quality by using audit-ready documentation, with all necessary quality metrics required for a smoother FDA inspection.
- Improving Cost Efficiency
Drug manufacturers don’t have to spend valuable resources on data collection, reporting, or audit preparation anymore. Automated systems on the platform free up productive time, which can be resourced towards process improvement and innovation.
- Competitive Edge in The Pharma
By adopting a proactive quality management solution such as CaliberMetrix, manufacturers not only optimize their business operations but also set new quality leadership benchmarks. This enhances their brand reputation in the marketplace and establishes trust with stakeholders, which translates into improved product quality and patient safety.
Looking at The Future: Industry 4.0 And The Next Era of Compliance
The transition from reactive compliance to proactive quality management is no longer optional for pharmaceutical manufacturers – it’s a necessity. A seamless integration with existing systems while offering a comprehensive suite of tools for continuous monitoring, data analysis, and reporting, CaliberMetrix paves the way for a more proactive, efficient, and compliant future in pharmaceutical manufacturing.
Are you ready to transform your approach to FDA compliance and quality management? Request a demo of CaliberMetrix today and see how real-time data can elevate your operations and ensure compliance.




