What’s Inside
Toggle‘Every 3 Hours, the FDA Issues a 483, Will Yours Be Next?
In FY 2024 alone, the FDA issued over 3,000 Form 483s, of which more than 60% were related to inadequate procedures, poor documentation practices, and quality system lapses.
What are the 483 observations?
FDA Form 483 observations identify non-compliance areas during drug, medical device inspections, and other regulated facilities. Knowing the most common observations and taking strong corrective action is crucial to ensuring compliance and preventing regulatory escalation.
For pharma leaders, these are not just regulatory red flags; they’re business disruptors. From batch holds to product recalls, each 483 observation has the potential to spiral into a costly warning letter or even import bans. Yet, many companies continue to react after the damage is done, scrambling to respond under pressure, with reputation and market access on the line.
The real question is: Are you equipped to respond to 483s with speed, clarity, and confidence? Or are you risking compliance fatigue and inspection repeatability?
When is an FDA Form 483 issued?
An FDA Form 483 is issued to firm management at the conclusion of an inspection when an investigator (s) has observed any conditions that, in their judgment, may constitute violations of the Food, Drug, and Cosmetic (FD&C) Act and related Acts._FDA
What steps should a company take after receiving an FDA 483 or warning letter to achieve compliance?
Its response requires a thorough understanding of the issues raised, a structured response, and a commitment to corrective action within a specified time frame. Let’s look at the right approach top-performing pharma companies are using to turn 483s into opportunities to build stronger, more resilient quality systems.
How to Compose an Effective Response
When the FDA points out potential violations in your operation, the right approach is to stay calm, listen carefully, acknowledge the issue, and respond responsibly. Remaining composed, cooperative, and corrective in your approach is your best strategy.
An effective response to an FDA 483 is essential to show your organization’s commitment to compliance and quality. Here’s how to draft an effective response:
What to do:
- Act promptly- Respond within 15 days: Your written response should be submitted within 15 business days of receiving the FDA 483. Timely responses are given priority before the FDA decides on further action.
- Address and outline corrective actions: Address each observation separately and clearly. Restate the observation, describe your understanding, and outline your corrective actions in a logical, easy-to-follow format.
- Show commitment: Show acknowledgment of the issues and commitment from senior management. Include a statement that your organization takes the findings seriously and is dedicated to remediation.
- Have Evidence of Correction: Submit supporting records for already completed actions, e.g., updated SOPs, training certificates, validation reports, or batch records to show completion. For not-yet-complete actions, submit a detailed plan with milestones and timelines.
What NOT to Do
- Do not neglect or procrastinate.
- Do not give unclear, generic, or generalized answers.
- Do not make blanket statements with no specifics.
- Do not argue observations without supporting data or regulatory rationale.
- Avoid omitting documentation or not including timelines for corrective measures.
- Avoid blaming inspectors or belittling the importance of findings.
How to avoid future FDA 483 citations – RCA and CAPA method
Root Cause Analysis (RCA)
Careful root cause analysis is necessary to identify the root cause for each observation and not merely the symptoms. Good RCA avoids recurrence and shows the FDA that your company knows and fixes systemic problems.
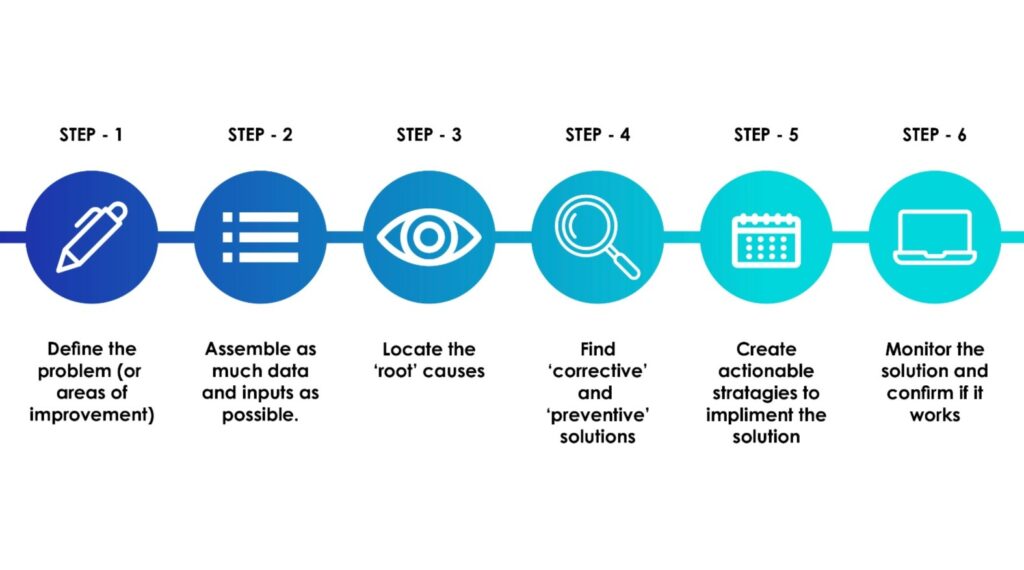
Corrective and Preventive Actions (CAPA)
CAPA is also a critical part of any quality system. Its purpose is to gather and analyze data, identify product or process issues, and implement actions that correct problems and prevent them from happening again.
Effective CAPA goes beyond quick fixes and targets root causes, verifies that fixes work, and documents the entire process. It also ensures teams are informed and that leadership has visibility. A strong CAPA system shows regulators that you’re not just reacting but have full control of long-term quality.
How to Create an Effective CAPA Plan
- Immediate Correction: Record any immediate containment or corrective actions taken.
- Root Cause Identification: Apply structured tools (e.g., 5 Whys, fishbone diagrams) to analyze and record the actual root cause for each observation.
- CAPA Development: For each root cause, create specific corrective actions (to repair the existing issue) and preventive actions (to avoid recurrence). Set ownership, timelines, and necessary training.
- Verification: Add a way to verify and track the effectiveness of the CAPA, for example, follow-up audits or metrics.
- Documentation: Document completed activities and a plan for future planned activities.
Changes at an organizational level – Importance of the QA Team
Endorsement and support from senior management are required, with resource allocation and organizational commitment. The Quality Assurance unit must monitor the CAPA process, track implementation, and perform follow-up checks to ensure efficacy.
- Preventive Strategies
- Building a Culture of Compliance and encouraging an organizational culture where compliance is valued at all levels.
- Foster transparent communication regarding quality issues and enable employees to raise concerns without fear of retaliation.
- Offer frequent training in regulatory guidelines and internal SOPs.
- Internal Audits and Mock Inspections
- Perform regular internal audits to detect and address compliance deficiencies actively ahead of FDA inspections.
- Employ mock inspections to simulate FDA visits, assess your team’s preparedness, and improve your response mechanisms.
- Use past FDA 483s and warning letters as a learning experience to build your compliance stance.
Introducing epiq by Caliber: Best to Avoid a 483 Observation
With over 3,000 FDA 483s issued in just one fiscal year, the question is no longer whether your organization will face scrutiny or not, but question it, when. Will you be prepared? Or will you be caught off guard, scrambling to protect your license, your product, and your reputation? Your response will define not just your compliance status, but your company’s future.
epiq by Caliber is a digital quality and compliance platform designed to strengthen pharmaceutical organizations’ regulatory readiness. It enables proactive monitoring, structured responses, and robust corrective actions, helping companies prevent and effectively manage FDA 483 observations
How it helps in avoiding FDA 483 observations, the key benefits
- Real-time Compliance Tracking: Monitors processes continuously to detect deviations early before they escalate into 483 observations.
- Automated Documentation: Ensures accurate, complete, and timely records that meet FDA expectations, reducing risks of poor documentation findings.
- Structured CAPA Management: Provides tools for root cause analysis and corrective/preventive actions, demonstrating systemic problem-solving to regulators.
- Audit Readiness: Maintains organized, validated records that can be presented confidently during FDA inspections.
- Risk Mitigation: Identifies compliance gaps proactively, helping companies address them before they trigger regulatory red flags.
- Management Visibility: Offers dashboards and reports that keep leadership informed, ensuring accountability and commitment to compliance.
- Culture of Compliance: Encourages transparency, training, and continuous improvement across teams, reducing repeat observations.
In Conclusion
epiq by Caliber empowers pharma companies to move from reactive firefighting to proactive compliance, minimizing the likelihood of FDA 483 observations and strengthening long-term regulatory trust.
Keep in mind, the FDA isn’t only focused on how you plan to address the specific issues they pointed out. They want to see that you understand the root causes and are working to fix the system weaknesses that allowed those problems to happen in the first place. Know more




