What’s Inside
ToggleQuality is paramount in Pharma 4.0, with increased quality standards, strict compliance measures, and high safety requirements from the regulated industry. With the highly evolving market trends, organizations are pacing toward digital readiness and quality assurance. Manufacturers are highly vigilant about quality checks during the manufacturing process to ensure they deliver safe products to the market. Thus, Manufacturing excellence is challenging with strict adherence to the highest quality standards.
Quality control in the pharma industry
As pharmaceutical companies directly impact patient health and safety, they are highly under the microscopic lens for the quality and efficacy of their products. According to the WHO, quality control (QC) is a part of the Good Manufacturing Processes (GMP), which ensures that the relevant tests are carried out and the products are not released for sale until the necessary process quality checks have been passed.
Key Stages of IPQC in Pharmaceutical Manufacturing
With high-quality adherence expectations from pharma manufacturers, quality control measures fall short of their capabilities. Quality control costs include possible human errors, equipment failures, abnormal interpretations, and missed analysis/maintenance schedules.

Fig. 1: Quality checks in manufacturing
As the name clearly states, IPQC is in-process quality control, which refers to quality checks during different stages of the drug manufacturing process. It starts from the raw material quality checks to the equipment, production environment, and packaging material. IPQC is regulated, and timely checks are made that identify any quality mishap in the drug production process and prevent it from affecting the next stage.
Why is IPQC important?
IPQC stands as an important pillar for quality adherence in the pharmaceutical industry. Since drugs are lifesaving, they hold a greater responsibility to have the right shape, right content, right amount, and the right form.
IPQC is important for pharmaceuticals –
- To detect any loopholes in the drug production process
- To provide real-time monitoring insights
- To enable a quick response to identified issues
- To enforce guidelines for the drug manufacturing process and packaging standards
IPQC equips a defined set of checks for different drug forms such as oral solid dosages, liquids, and sterile products.
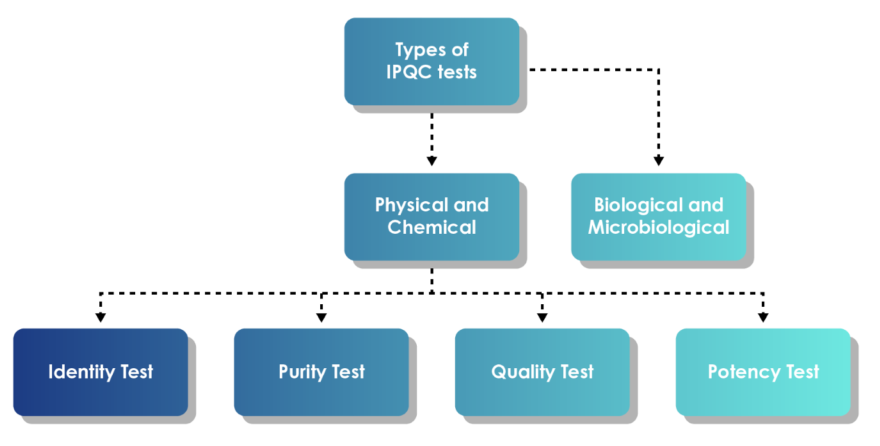
Fig. 2: Types of IPQC tests
Challenges with IPQC – Is There Still a Manual Dependency?
A single test result can bring up a report of 200-300 pages, depending on the number of batches produced. Maintaining time and test details thus becomes cumbersome and resource-intensive. Manual handling of large report sets leads to missed samples, data inconsistencies, scalability failures, restricted report access, and facility inefficiencies. However, in process quality control, checks are usually frequency-based, where analysts schedule sample analysis to conduct these tests and record observations.
The FDA needs documentation to approve the activities performed, test accuracy, and actions taken. If it is not documented, it has not happened for the regulatory agency.
Digitalization of the IPQC process for better tracking, traceability, and efficiency
Digitalizing process quality control tests accelerates the process, ensuring accuracy and efficiency. Electronic IPQC checks ensure there are no missed sample analysis schedules, maintenance alerts, traceability, and audit compliance. With n number of batches produced every day, pharmaceuticals need a digital IPQC system to justify the purpose with precision.
Introducing CaliberIPQC – Your Driver for Manufacturing Excellence
CaliberIPQC is designed to address the modern-day IPQC challenges. It monitors, controls, and improves manufacturing efficiency at every stage. A robust solution for administering 100% control over IPQC tests, seamless in eliminating manual intervention, to allow users to have complete control over product quality with consistent, automated, digitalized in-process quality checks. With,
- Streamlined sample analysis schedules
- Establish predefined test protocols as per business requirements and SOPs.
- Reduced duplication of tasks.
- Easy access to the test results on any system, even tablets.
- Generate trend analysis reports of the IPQC data at any time.
The In Process Quality Control module in CaliberIPQC generates and triggers calibration schedules automatically based on the set frequency. Integrating port-based IPQC instruments using a standard RS232 port, such as the balance, friability, hardness, thickness, moisture analyzer, and others, data automatically gets registered in the system. It minimizes the errors and discrepancies in the overall process and promotes data updates in real time to strengthen and speed up decision-making.
If Manufacturing Excellence is your goal, CaliberIPQC must be your shopfloor partner.
Click here to learn more about how CaliberIPQC has helped Zydus, Strides, like big pharma companies to streamline their in-process quality checks.




