Highly regulated industries have the responsibility of ensuring data integrity in the entire lifecycle of documentation. As per Food and Drug Administration (FDA), European Medicines Agency (EMA), Medicines and Healthcare items Regulatory Agency (MHRA), World Health Organization (WHO), and other regulatory bodies, data has no value if it is not preserved properly.
Maintaining data integrity in Pharmaceutical industry is crucial for several reasons. Documents if saved properly ensure data integrity. In turn, data integrity ensures recoverability, accessibility, traceability, and availability of data anytime for any purpose. Data helps in finetuning quality processes, finding current and future trends of production, analyzing quality of products, and driving business decision making. But raw data needs to undergo different formats to facilitate informed decisions. As data integrity is top priority for industries to produce quality products, regulatory agencies like FDA inspect and issue 483 forms and warning letters to industries that do not ensure data integrity. For data integrity, WHO made simple ALCOA guidelines that every regulated industry needs to follow to keep their data safe, readable, reliable, and retrievable.
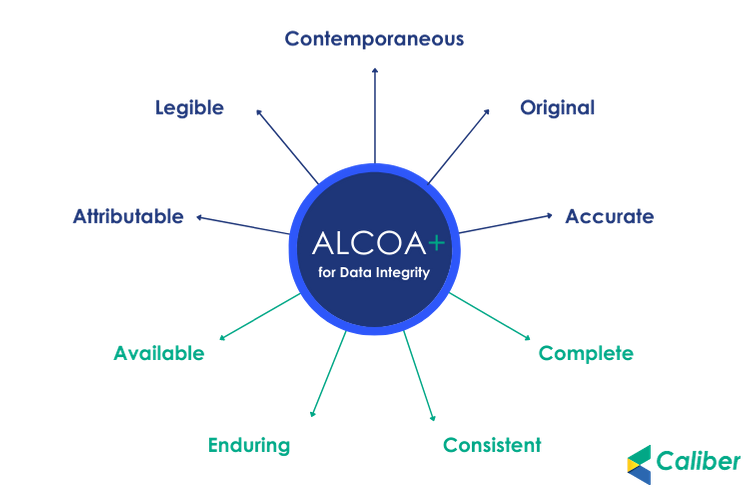
ALCOA is short for the essential characteristics of good documentation – Attributable, Legible, Contemporaneous, Original, and Accurate.
The ALCOA guidelines put down by good documentation guidelines are applied to all forms of documentation and evidence maintained such as paper, electronic, and hybrid.
The general regulations around data integrity were in place since 1978. But the acronym ALCOA came into existence in the 1990’s. Since then, the industry has changed dramatically. The generic drug industry developed tremendously, forming more than 80% of prescription medicine in United States alone. Along with it, the development of biosimilars, virtual organizations, contract manufacturing companies, advancements in automation and information technology, and globalization of the industry resulted in the need for re-evaluation of what data integrity means. This now applies to all documentation, regardless of whether it is produced from electronic, paper-based, or hybrid systems.
Further, the number of citations from regulatory bodies such as FDA, EMA, MHRA, WHO, and others have also increased internationally. All these changes and an increase in data integrity related violations have led to the revival of interest and need to reeducate the industry on the fundamental principles and guidelines regarding the proper control of data. It is an essential step as it deals with the quality and safety of life-saving medicines.
As an extension to the ALCOA guidelines, a few more attributes must now be scrupulously followed to ensure data security and integrity.
Data integrity is a crucial factor that can turn tables for any regulated industry if not properly maintained. With automation and technological advancements, many tools & applications can now help the industry safeguard the integrity of their data.
Ensuring and achieving data integrity is simple with Caliber’s informatic systems. Caliber systems generate and collate data in full compliance with ALCOA +. With CaliberLIMS, the complete laboratory information and details can be electronically recorded, retrieved, and used for future analysis. CaliberDMS helps in ensuring GDP, GMP, and validation by focusing on good documentation practices as suggested by ALCOA Plus guidelines. CaliberAPQR makes annual product quality reviews and audit work simple. Similarly, other Caliber products provide a holistic approach to address data integrity. External auditors can be assured that the documents created or collected in the form of evidence through Caliber systems are valid and true for audit review.
Choosing ALCOA Plus compliant Caliber products is a simple way of achieving data integrity and ensuring enhanced product quality. Click here for a free product demo.